About the Vaccines
Four COVID-19 vaccines (Pfizer, Moderna, Johnson & Johnson, and Novavax) are authorized or approved for use in the United States to prevent COVID-19. Read about the approved and authorized vaccines.
If you have already completed your vaccine series and are interested in a booster dose of the COVID-19 vaccine, visit our additional doses page.
Find a COVID-19 vaccination location near you
Emergency Use Authorizations
Patient Fact Sheets
Vaccine Side Effects
Side effects are a normal reaction after receiving any vaccine. Mild side effects such as muscle soreness at the injection site, being tired, and even fever are common. It’s a sign that that the vaccine is working in your body. But not everyone experiences side effects and that’s okay too. Learn more information about side effects from the CDC.
Serious side effects from the COVID-19 vaccine, although rare, have been known to occur. If you have any health problems after vaccination, report them to the Vaccine Adverse Event Reporting (VAERS).
Seek medical care if you think you or your child have any of these symptoms within a week after COVID-19 vaccination. Contact your health care provider if you have questions.
Symptoms include:
- Chest pain
- Shortness of breath
- Feelings of having a fast-beating, fluttering, or pounding heart
The most serious results of side effects includes:
- Myocarditis and pericarditis
- GBS (Guillain-Barré Syndrome)
- Thrombocytopenia Syndrome (TTS)
Vaccine Safety & Monitoring
 Learn more about how the U.S. ensures COVID-19 vaccines are safe for the American public:
Learn more about how the U.S. ensures COVID-19 vaccines are safe for the American public:
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety.html
- COVID-19 vaccines were developed using science that has been around for decades.
- COVID-19 vaccines are safe—much safer than getting COVID-19.
- COVID 19-vaccines are effective at preventing severe illness from COVID-19 and limiting the spread of the virus that causes it.
Millions of people in the United States have received COVID-19 vaccines under the most intense safety monitoring in U.S. history.
Is it OK for Me to Get the Vaccine?
Vaccine Ingredients and Allergy Information (7.30.21)| Español
COVID-19 Vaccine Safety Monitoring Systems for Pregnant People
Learn how CDC is monitoring the safety of COVID-19 vaccination in people who are pregnant.
V-SAFE Vaccine Safety Monitoring
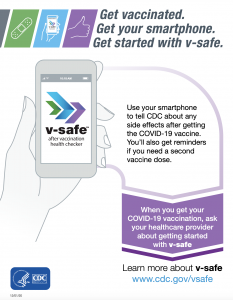 (V-SAFE) Your after vaccine health Checker – Use your smartphone to tell CDC how you’re feeling after your COVID-19 vaccination and personalized health check-ins to make sure you’re doing well.
(V-SAFE) Your after vaccine health Checker – Use your smartphone to tell CDC how you’re feeling after your COVID-19 vaccination and personalized health check-ins to make sure you’re doing well.
Vaccine Adverse Events Reporting System (VAERS)
VAERS is the national system that collects reports of adverse events that happen after vaccination.
How to Report Adverse Events to VAERS:
There are two ways to submit a report to VAERS:
Option 1:
Submit a VAERS Report online (Preferred)
The online VAERS Report must be completed and submitted in the same session; it cannot be saved and edited at a later time.
Note: sessions time out after 20 minutes of inactivity; no information is saved.
Option 2:
Download a Writable PDF Form and upload when ready
The Writable PDF Form can be downloaded and completed electronically on your own time. When ready, return to the VAERS Writable PDF web page (use link above) and follow Step 2 instructions to upload the form.
 More information on reporting an adverse event to VAERS. If you need further assistance, please email info@VAERS.org or call 1-800-822-7967.
More information on reporting an adverse event to VAERS. If you need further assistance, please email info@VAERS.org or call 1-800-822-7967.
More Information about VAERS
Important Links
- Information on Additional Doses
- Frequently Asked Questions About COVID-19 Vaccines and Boosters
- FDA COVID-19 Vaccine Information
- Path from Vaccine to EUA infographic
- FDA: Vaccine Development 101
- FDA: Emergency Use Authorization for Vaccines Explained
Find My Vaccine
Find your COVID-19 Vaccine
Stats on COVID-19
For more data on Delaware COVID-19 cases including demographic breakdowns, go to My Healthy Community